Sodium-ion batteries (SIBs) are increasingly regarded as a promising alternative to lithium-ion batteries in electrochemical energy storage due to their cost-effectiveness, abundant resource availability, and excellent low-temperature performance. However, despite these benefits, the commercial deployment of SIBs is hindered by significant safety concerns. Battery safety incidents are predominantly attributed to thermal runaway (TR), which can damage individual cells and trigger cascading failures within the battery module. This escalation can result in widespread TR propagation, posing a severe threat to the integrity of energy storage systems. Consequently, a comprehensive investigation into the TR mechanisms of SIBs is essential for improving the fire safety of sodium-based energy storage systems.
Recently, Prof. Qingsong Wang from SKLFS conducted a comprehensive investigation into the TR behavior of large-format commercial SIBs across different states of charge (SOCs). This study provided a more in-depth analysis than previous research by examining heat generation, gas production, and mechanical changes occurring in SIBs during TR. The findings indicate that as the SOC increases, the proportion of H₂ in the released gases rises significantly. At 100% SOC, H₂ constitutes 42% of the emitted gases, with a flammability range of 6.5% ~ 69.0%, highlighting the increased explosion risk of SIBs under high SOCs. Furthermore, SIBs generate a substantial amount of heat during TR, resulting in the ejection of hot particles as sparks. Nevertheless, the violent gas release facilitates heat dissipation inside the battery and isolates combustible gases from sparks and oxygen, ultimately leading to the self-extinguishing behavior of SIBs. The associated work has been published in the Energy Storage Materials (77 (2025) 104197).
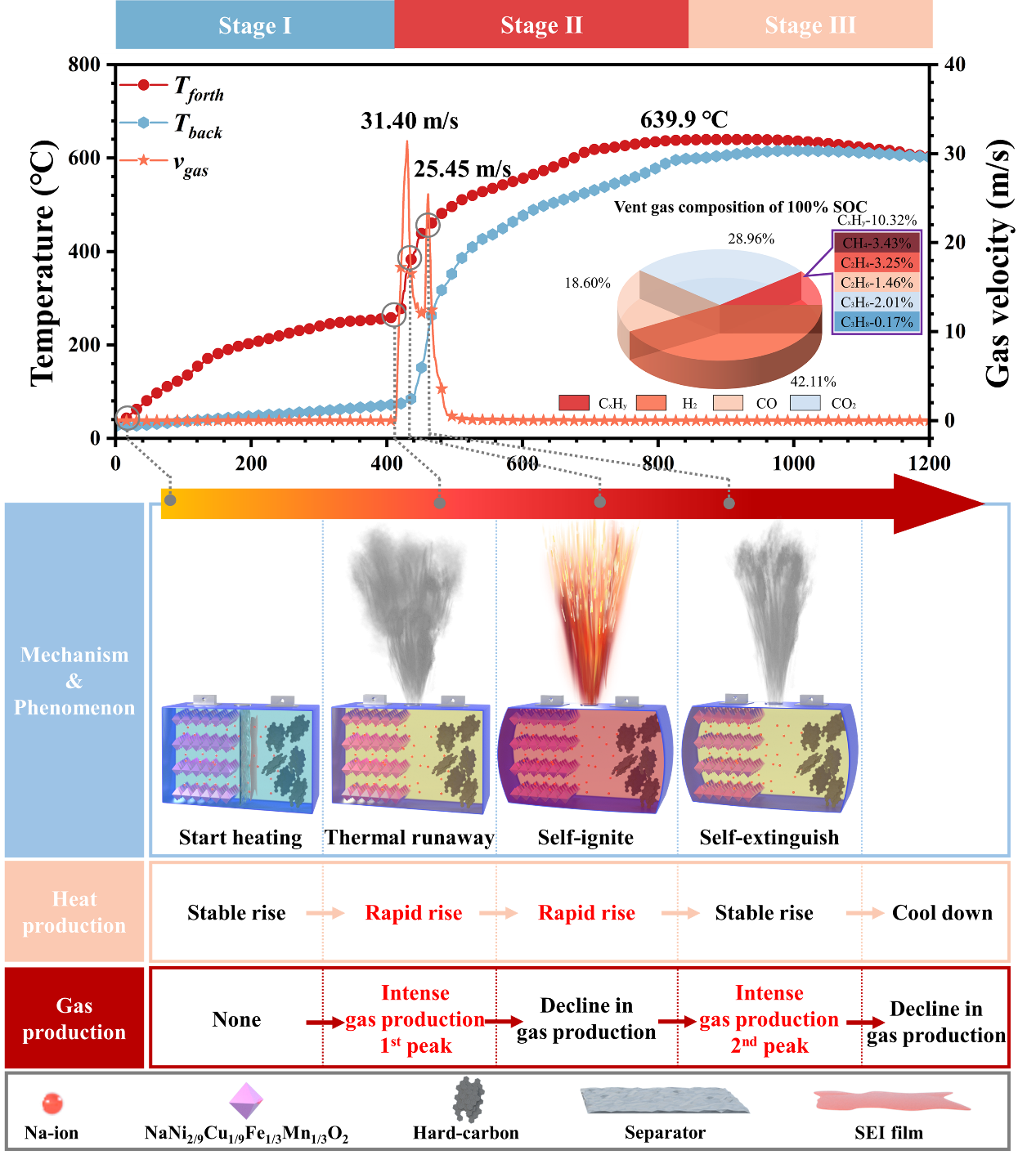
Fig. 1. Thermal runaway mechanisms of SIBs